The molecular structure of Andrastin A
Click on the image above to interact with the 3D model of the Andrastin A structure
Andrastin
Discovery, producing organism and structure (1-4)
Andrastins were isolated from the culture broth of the fungal strain FO-3929 and found to be protein farnesyltransferase inhibitors. The absolute configuration of the p-bromobenzoyl derivative of andrastin A was elucidated by X-ray crystallographic analysis and its skeleton was identified as ent-5α,14β-androstane.
Physical data (Andrastin A)
White powder. C28H38O7; mol wt 486.60. Sol. in DMSO, MeOH, CHCl3. Insol. in H2O, hexane.
Biological activity (2,4,5)
1) Inhibition of protein farnesyltransferase (2,4)
Protein farnesyltransferase catalyzes the post-translational
modification of ras p21 that is obligatory for cell transformation of this oncogene protein.
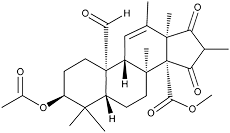
Andrastin A
2) Enhancement of drug accumulation in vincristine-resistant KB cells (5)
Andrastin A enhanced the cytotoxicity of vincristine 1.5–20 fold in vincristine-resistant KB cells (VJ-300).
No antimicrobial activity was shown at a concentration of 1,000 μg/ml against Xanthomonas oryzae KB88, Candida albicans KF-1, Saccharomyces cerevisiae KF26, Mucor racemosus KF223 (IFO 4581), Pyricularia oryzae KF180, Aspergillus niger KF 103 (ATCC 6275), Staphylococcus aureus KB34 (FDA 209P), Bacillus subtilis KB27 (PCI 219), Escherichia coli KB8 (NIHJ), E. coli KB176 (NIHJ JC-2), Pseudomonas aeruginosa KB105 (P3), Micrococcus luteus KB40 (PCI 1001), Bacteroides fragilis KB169, Mycobacterium smegmatis KB42 (ATCC607), or Acholeplasma laidlawii KB174 (PG8).
Biosynthesis (3)
The incorporation study of single and double labeled acetate into andrastin A and the result of a biosynthetic study of citreohybridonol, an analog of andrastins, conducted by Kosemura et al.,(6) suggested that andrastins were synthesized as follows. A sesquiterpene, synthesized from farnesyl pyrophosphate, is cyclized to form an enantiomer of drimane. 3,5-Dimethylorsellinate derived from tetraketide is combined with drimane to form ring C. Ring D changes from cyclohexane to cyclopentane by a rearrangement. Thus, an enantiomer of the 5α,14β-androstane skeleton is formed. Though many compounds produced by fungi from farnesylate and orsellinate have been reported, their absolute structures have not been elucidated. The absolute structures may therefore be different from the reported ones.
References
1. [605] K. Shiomi et al., Tetrahedron Lett., 37, 1265–1268 (1996)
2. [614] S. Ōmura et al., J. Antibiot., 49, 414–417 (1996)
3. [615] R. Uchida et al., J. Antibiot., 49, 418–424 (1996)
4. [650] R. Uchida et al., J. Antibiot., 49, 1278–1280 (1996)
5. [690] M.-C. Rho et al., J. Antibiot., 51, 68–72 (1998)
6. S. Kosemura et al., J. Chem. Soc., Perkin Trans. 1, 135–139 (1994)


