Staurosporine: world’s first indolocarbazole & anticancer drug precursor
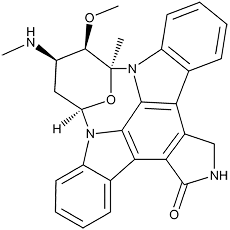
Staurosporine
In terms of the development of current anti-cancer agents, staurosporine represents one of the world’s most significant advancements. This remarkable chemical was discovered at the Kitasato Institute in 1977 while screening for microbial alkaloids using a recently introduced innovative Physico-Chemical system.
Staurosporine, the world’s first indolocarbazole compound, was isolated from a culture of Streptomyces staurosporeus found in a soil sample collected in Mizusawa City, Japan. Our initial assays demonstrated that it possessed promising antifungal and hypotensive properties but lacked any antibacterial bioactivity.
Of greater significance, almost 10 years after it’s discovery, staurosporine was shown to be a nanomolar inhibitor of protein kinases. Protein kinases regulate essential aspects of cells, including metabolism, cell cycle progression, cytoskeletal arrangement and cellular replication. Staurosporine is an extremely potent - but non-specific - inhibitor of protein kinases, particularly tyrosine kinases, and has a potent cytotoxic effect on cancer cells. These findings stimulated the global pharmaceutical industry to search for exploitable protein kinase inhibitors through the screening of natural chemicals as well as via chemical synthesis, with staurosporine consequently being widely recognized as precursors of many of today’s highly successful anticancer chemotherapeutic agents.
Staurosporine is a large highly hydrophobic molecule. Its indolocarbazole structure is derived from two molecules of tryptophan, with the sugar moiety being derived from glucose and methionine. The cleft at the domain interface is highly hydrophobic, such that large complementary surface areas surround the bisindolylcarbazole ring system of bound staurosporine. Staurosporine overlaps extremely well with the adenosine group of ATP, the lactam ring mimicking the hydrogen-bonding interactions of adenine, while the glycosyl group mimics those of ribose so, not surprisingly, it acts as a non-selective protein kinase inhibitor.
Over the past 30 years, indolocarbazole compounds have been isolated from a variety of organisms, including bacteria, fungi and invertebrates. The unusual molecular architecture of the indolocarbazoles, coupled with their excellent biological activity, made them of primary interest to cancer researchers. After 1986, development of small-molecule kinase inhibitors quickly became one of the most widely and intensely pursued areas of drug discovery worldwide.
In the 1990s, imatinib, a Bcr-Abl tyrosine kinase inhibitor, was synthesized and, following human clinical trials against chronic myelogenous leukemia, it became the first kinase inhibitor approved for use in 2001. In 1992, mammalian topoisomerases were shown to be targets for indolocarbazoles, opening up new possibilities in that indolocarbazole compounds could selectively interact with ATP binding sites of not only protein kinases but also other proteins that had variations in ATP-binding sites. Today, approaching 30 kinase inhibitors have already been registered for use as anti-cancer agents.
As a measure of the fundamental and on-going impact of staurosporine, >3,000 compounds, active against a wide range of protein kinases, are currently under preclinical investigation for various cancers, ophthalmic diseases, central nervous system disorders, osteoporosis and other ailments, with >130 novel tyrosine kinase inhibitors undergoing clinical trials.
As an indication of the elemental and radical importance of staurosporine, in both industry and academia, >1 million kinase research papers have been published, >5,000 crystal structures of kinases have been identified, plus inhibition assays have been developed for over 80% of the human kinome. Around one-third of all protein targets currently under investigation in the pharmaceutical industry are either protein or lipid kinases, and kinase inhibitors now account for a major portion of all newly-approved drugs.
Recently, staurosporine has displayed promise in tackling parasites, with parasite signalling pathways now attracting increasing attention as possible drug targets. Protein kinases are essential in the growth and proliferation of malarial parasites and tuberculosis mycobacteria, while apoptosis in single-cell parasites is also being investigated. Critical roles for protein kinases in the growth and infectivity of trypanosomatid parasites have already been identified. Staurosporine is known to induce cell death in Trypanosoma brucei and substantial drug development programmes are now focussing on staurosporine-class antiparasitic drugs. In addition, it has recently been reported that staurosporine possesses insecticidal properties, inducing apoptosis in lepidopteran Sf9 cell lines, and it is being suggested that staurosporine has potential for use as an insecticide against lepidopteran agricultural pests.

