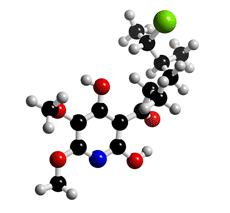
The molecular structure of Atpenin A4
Click on the image above to interact with the 3D model of the Atpenin A4 structure
Atpenin
Discovery, producing organism and structure (1-3,5)
Atpenins were isolated from the culture broth of the fungal strain FO-125. Atpenins inhibited growth of both fatty acid synthase (FAS) deficient (A-1) and acyl-CoA synthetase I (ACS-I) deficient (L-7) mutants of Candida lipolytica, and also inhibited incorporation of long chain fatty acids into lipid fractions in Raji cells. The absolute configuration of atpenin A4 was confirmed by X-ray crystallographic analysis(2). The first total synthesis of atpenin B was reported by Quéguiner et al(3). Recently, atpenins and their structurally related compound, harzianopyridone, have been recognized to have potent mitochondrial complex II (succinate-ubiquinone oxidoreductase) inhibitory activity(5).
Physical data (Atpenin A5)
White powder. C15H21NO5Cl2; mol wt 366.24. Sol. in DMSO, MeOH, EtOH, CHCl3. Insol. in H2O.
Biological activity (1,4,5)
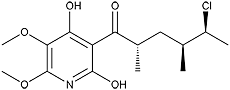
Atpenin A4
1) Antimicrobial activity (1)
2) Atpenin B decreased cellular ATP levels in Raji cells with an IC50 value of 20 nM, suggesting inhibition of an ATP-generating system by the drug (4).
3) Atpenins inhibited nematode and mammalian mitochondrial complex II (5).4) Atpenin A5 inhibited bovine heart succinate-ubiquinone reductase much more potently than known complex II inhibitors (5).
5. Studies of carboxin resistant fungi indicate that amino acid residues close to the [3Fe- 4S] center in Ip, as well as a residue in CybS, located in the cytoplasmic loop between transmembrane segments, are important for carboxin binding and possibly for ubiquinone recognition. Thus, ubiquinone is thought to bind to complex II at the interface between Ip and the membrane-anchor domain. This explains the observation that atpenins affected not only succinate-ubiquinone reductase (SQR) activity but also succinate dehydrogenase (SDH) activity of the bovine heart complex II. Inhibition of SDH by atpenins was less potent than that of SQR, and complete SDH inhibition was not achieved even at high concentrations. These findings of mixed type inhibition of SQR activity are very similar to those reported for carboxin.
References
1. [405] S. Ōmura et al., J. Antibiot., 41, 1769–1773 (1988)
2. [454] H. Kumagai et al., J. Antibiot., 43, 1553–1558 (1990)
3. G. Quéguinier et al., J. Org. Chem., 59, 6173-6178 (1994)
4. [446] K. Oshino et al., J. Antibiot., 43, 1064–1068 (1990)
5. [823] H. Miyadera et al., Proc. Natl. Acad. Sci. USA, 100, 473–477 (2003)


