The molecular structure of Herbimycin A
Click on the image above to interact with the 3D model of the Herbimycin A structure
Herbimycin
Discovery(1), producing organism(1) and structure (2-7)
Herbimycins, new benzoquinoid ansamycin antibiotics, were isolated and acknowledged as herbicidal agents (1). The absolute configuration of herbimycin A was confirmed by X-ray crystallographic analysis (3). The first total synthesis of herbimycin A was reported by Tatsuta et al (4).
Physical data (Herbimycin A) (1)
Colorless powder. C30H42N2O9; mol wt 574.30. Sol. in MeOH, EtOH, CHCl3, EtOAc, DMSO, acetone, DMF, benzene. Insol. in H2O, Et2O, hexane.
Biological activity (2,4,5)
1) Herbicidal activity of herbimycin A (1,5)
2) Anti-tobacco mosaic virus activity (5)
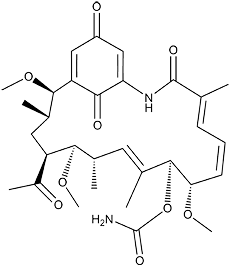
Herbimycin A
4) Effects on growth and differentiation of human erythroleukemia cells (9)
5) Chemical modification and antitumor activity (10,11)
Several halogenated and other related derivatives have been synthesized and evaluated
in vivo for their activities against Ehrlich ascites carcinoma. Some of these derivatives
showed higher in vivo activity than herbimycin A.
6) Inhibition of tyrosine kinase activity (12)
Since herbimycin A appears to exhibit specific inhibitory activity against tyrosine kinases
in cells, this antibiotic is beneficial in determining whether tyrosine phosphorylation is
involved in the mechanisms for cell transformation, growth, and differentiation.
7) Mechanism of antiproliferative and antitumor (13,14)
Herbimycin A specifically inhibits the cytosolic chaperone HSP 90 and its endoplasmic
reticulum (ER) homologue GRP 94. This compound has antiproliferative and antitumor
effects: as it binds to HSP 90, it inhibits the HSP 90-mediated conformational maturation/refolding reaction, hereby promoting degradation of HSP 90 substrates.
References
1. [153] S. Ōmura et. al., J. Antibiot., 32, 255-261 (1979)
2. [166] S. Ōmura et. al., Tetrahedron Lett., 44, 4323-4326 (1979)
3. [186] A. Furusaki et. al., J. Antibiot., 33, 781-782 (1980)
4. K. Tatsuta et al., Tetrahedron Lett., 32, 6015 (1991)
5. [193] Y. Iwai et. al., J. Antibiot., 33, 1114-1119 (1980)
6. [207] A. Nakagawa et. al., J. Chem. Soc. Jpn., Chem. & Ind. Chem., 892-894
(1981)
7. [356] K. Shibata et. al., J. Antibiot., 39, 1630-1633 (1986)
8. [397] Y. Uehara et. al., J. Antibiot., 41, 831-834 (1988)
9. [479] Y. Honma et. al., Anticancer Res., 12, 189-192 (1992)
10. [298] S. Ōmura et. al., J. Antibiot., 37, 1264-1267 (1984)
11. [338] K. Shibata et. al., J. Antibiot., 39, 415-423 (1986)
12. Y. Uehara et. al., Methods Enzymol., 201, 370-379 (1991)
13. Pratt, W. B. et. al., Proc. Soc. Exp. Biol. Med. 217, 420-434 (1998)
14. Lia, Supin-Rosa et al. J. Biol. Chem. 275, 21850-21855 (2000)


