The molecular structure of Lactacystin
Click on the image above to interact with the 3D model of the Lactacystin structure
Lactacystin
Discovery, producing organism and structure (1-3, 12-14)
Lactacystin was originally found while screening for neurite outgrowth inducers in Neuro 2a, a cell line of murine neuroblastoma cells. The absolute configuration of the structure was confirmed by X-ray crystallographic analysis (2). The total synthesis of lactacystin has been reported by several groups.
Physical data
Colorless crystal. C15H24ON2O7S; mol wt 376.13. Sol. in H2O, MeOH, DMSO, pyridine. Insol. in benzene, EtOAc,
CHCl3.
Biological activity (4-8)
1) Lactacystin inhibits cell cycle progression (4) and induces neurite outgrowth in Neuro 2a cells (1).
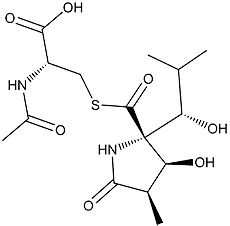
Lactacystin
2) Lactacystin inhibits proteasome activity (5). Proteasome exhibits three protease activities, of the three, lactacystin inhibits chymotrypsin-like activity most potently, followed by trypsin-like activity, but its inhibitory activity against peptidylglutamylpeptide hydrolytic activity is relatively weak.
Lactacystin is specifically bound to the N-terminal threonine residue of certain β subunits (subunit X for mammalian cells (5) and subunit β5 for yeast (7)). Recently, it has been proposed that lactacystin is converted non-enzymatically to the β-lactone derivative (Omuralide), which reacts directly with the threonine active site.
Structure–activity study (3)
Lactacystin derivatives were evaluated in neurite outgrowth of Neuro 2a cells and growth inhibition of osteosarcoma cells. The same derivatives were found to elicit both activities, suggesting that they are based on the same mechanism. The presence of a hydroxyisobutyl group on C-5 and the 9S configuration are important, as is the 6S,7R configuration of the γ-lactam ring, revealing specific structural requirements for activity. The most significant finding was that clasto-lactacystin β-lactone (later designated Omuralide by Prof Corey), shows almost the same activity as lactacystin (9).
Application
Lactacystin is recognized as the most specific inhibitor of proteasome due to its mode of
action. Therefore, lactacystin has been used to reveal and support the cellular proteasome
functions.
1) Cell cycle: [571], [642], [667], [719], [735]
2) Apoptosis: [599], [676], [691], [692], [713], [716], [739]
3) Immunoantigen processing: [666], [667]
4) Protein degradation on ER: [593]
5) Receptor degradation: [594], [606], [653]
6) Transcription factor degradation: [643], [674], [769],
7) Other regulatory protein degradation: [618], [627], [641], [654], [670], [678], [698],
[704], [752], [756], [770], [771], [780], [783], [818]
8) Anti-tumor: [750],
9) Anti-malaria: [830]
10) Anti-angiogenesis: [697]
The above brackets [ ] show references for the applications (See Ōmura Publications).
---
Lactacystin is commercially available from Kyowa Medex.
References
1. [455] S. Ōmura et al., J. Antibiot., 44, 113–116 (1991)
2. [456] S. Ōmura et al., J. Antibiot., 44, 117–118 (1991)
3. [760] H. Tomoda et al.,Yakugaku Zasshi, 120, 935-949 (2000)
4. [571] M. Katagiri et al., J. Antibiot., 48, 344–346 (1995)
5. G. Fenteany et al., Science, 268, 726–731 (1995)
6. K. Tanaka et al. J. Leukocyte Biol., 56, 571 (1994)
7. M. Groll et al., Nature, 386, 463–471 (1997)
8. L. R. Dick et al., J. Biol. Chem., 272, 182–188 (1997)
9. E. J. Corey et al., Chem. Pharm. Bull., 47, 1-10 (1990)
10. [546] A. Nakagawa et al., Tetrahedron Lett., 35, 5009–5012 (1994)
11. [589] S. Takahashi et al., J. Antibiot., 48, 1015–1020 (1995)
12. [507] T. Sunazuka et al., J. Am. Chem, Soc., 115, 5302 (1993)
13. [510] T. Sunazuka et al., Tetrahedron Lett., 34, 4447–4448 (1993)
14. [628] T. Nagamitsu et al., J. Am. Chem, Soc., 118, 3584–3590 (1996)


