The molecular structure of Motilide
Click on the image above to interact with the 3D model of the Motilide structure
Motilide
Discovery, producing organism and structure (1,2,4-6)
Erythromycin A, a well-known macrolide antibiotic, showed gastrointestinal motor stimulating (GMS) activity. GMS activity is very similar to the effect caused by the hormone motilin. A series of erythromycin derivatives (especially EM 522, EM 523, EM536 and EM 574) were synthesized under unique reversed conception to search for a “motilide” defined as motilin-like macrolides with potent GMS activity.
Synthesis of motilides (2)
Biological activity (2,5-10)
1) Antimicrobial activity (MIC) and gastrointestinal motor stimulating (GMS) activity in
canine (in vivo) of motilides EM 523, EM 574 and EM 536.
2) Motilides inhibit the specific binding of 125I-motilin to duodenal muscle (3,10).
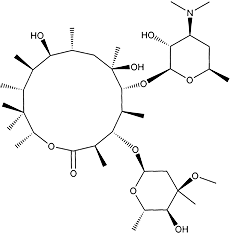
Motilide
3D structure-activity relationship between motilide (EM536) and motilin
We applied a rational computational procedure consisting of conformational analysis and a novel superposing method to investigate the 3D structure-activity relationship between motilide (EM536) and motilin. The HA9, DA10, and DA11 atoms for EM536 were superposed on the DA9, HD10, and HD11 atoms for motilin, respectively. We have proposed common 3D structural features between these molecules, which may be related to for their activity.
References
1. [333] S. Ōmura et al., J. Antibiot., 38, 1631-1632 (1985)
2. [373] S. Ōmura et al., J. Med. Chem., 30, 1941-1943 (1987)
3. [386] Y. Kondo et al., Biochem. Biophys. Res. Commun., 150, 877-882 (1988)
4. [403] Z. Itoh et al., Chemotherapy, 36, 104-115 (1988)
5. [426] K. Tsuzuki et al., Chem. Pharm. Bull., 37, 2687-2700 (1989)
6. [427] T. Sunazuka et al., Chem. Pharm. Bull., 37, 2701-2709 (1989)
7. [434] N. Inatomi et al., J. Pharmacol. Exp. Ther., 251, 707-712 (1989)
8. [441] S. Ōmura et al., In “Motilin” (Ed. By Z. Itoh) pp.245-256, Academic Press
(1990)
9. [451] T. Satoh et al., J. Pharmacol. Exp. Ther., 254, 940-944 (1990)
10. [557] M. Satoh et al., J. Pharmacol. Exp. Ther., 271, 574-579 (1994)


