The molecular structure of Pepticinnamin E
Click on the image above to interact with the 3D model of the Pepticinnamin E structure
Pepticinnamin
Discovery, producing organism and structure (1-3)
Pepticinnamins, consisting of 6 components, were isolated from a culture broth of the actinomycete strain OH-4652 and recognized as inhibitors of protein farnesyltransferase from an assay using a partially purified enzyme from human monocyte THP-1 (ATCC TIB 202). The structure of component E was elucidated. The stereochemistry of the amino acids was elucidated using chiral HPLC, with the exception of N-methyl-(2-chloro-3- hydroxy-4-methoxy)-phenylalanine. Its stereochemistry was revealed by total synthesis of the pepticinnamin E diastereomers (3).
Physical data (Pepticinnamin E)
White powder. C49H54N5O10Cl; mol wt 908.46. Sol. in DMSO, MeOH, EtOAc, CHCl3. Insol. in H2O, hexane.
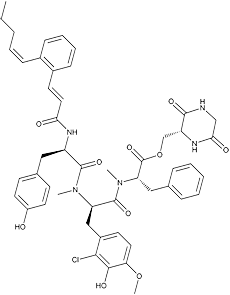
Pepticinnamin E
Biological activity (1,4,5)
1) Specific protein farnesyltransferase inhibition
Protein farnesyltransferase catalyses a post-translational modification of ras p21 obligatory for cell transformation of the oncogene protein.
2) Kinetic analysis of protein farnesyltransferase inhibition by pepticinnamin E
Pepticinnamin E inhibits protein farnesyltransferase competitively with respect to ras
p21 and noncompetitively with respect to farnesyl diphosphate (FPP).
References
1 [498] S. Ōmura et al., J. Antibiot., 46, 222–228 (1993)
2. [499] K. Shiomi et al., J. Antibiot., 46, 229–234 (1993)
3. K. Hinterding et al., Angew. Chem. Int. Ed. Engl., 37, 1236–1239 (1998)
4. [514] H. Takeshima and S. Omura, Tanpakushitsu Kakusan Koso, 38, 1695–1703
(1993)
5. [554] S. Ōmura and H. Tomoda, Pure Appl. Chem., 66, 2267–2270 (1994).


