The molecular structure of Staurosporine
Click on the image above to interact with the 3D model of the Staurosporine structure
Staurosporine
Discovery, producing organism and structures (1-6)
Staurosporine was originally isolated from a culture broth of the actinomycete strain AM-2282 while screening for microbial alkaloids. Later, it was found to show strong inhibitory activity against protein kinases (6). The structure and absolute configuration of staurosporine was elucidated by X-ray crystallographic analysis (2–4). The total synthesis of staurosporine has been reported by many groups. The first total synthesis was reported by Danishefsky et al (7).
Physical data
Pale yellow crystals. C28H26N4O3; mol wt 466.20. Sol. in DMSO, DMF. Slightly sol. in CHCl3, MeOH.
Biological activity (1,6,8)
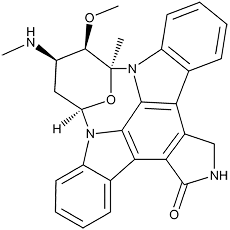
Staurosporine
1) Inhibition of protein kinase C (6)
IC50 =2.7 nM (rat brain)
2) Antimicrobial activity (1)
Staurosporine showed antimicrobial activity against fungi and yeast.
IC50 = 280 nM (1 hr-exposure), 4 pM (72 hr-exposure)
4) Acute toxicity (mice i.p.) (7)
LD50 =6.6 mg/kg
5) Inhibition of Ca2+-dependent contraction (8)
Staurosporine was found to be a potent relaxant of rabbit aortic strips contracted by various
agonists.
6) Kinetic analysis of protein kinase C inhibition by staurosporine
7) Chemical modification and antiangiogenesis effect
Staurosporine was focused on chemical modification of the amino sugar moiety. Of the new
derivatives reported (9), the most interesting compound is derivative 13, an antiangiogenic
agent.
---
Staurosporine is commercially available as a potent protein kinase inhibitor for biochemical research. Staurosporine analogues, 7-hydroxy-staurosporine(UCN01) and Nbenzoyl staurosporine (CGP 41251) are currently under clinical investigation as potential anticancer drugs (10).
References
1. [127] S. Ōmura et al., J. Antibiot., 30, 275–282 (1977)
2. [148] A. Furusaki et al., J. Chem. Soc., Chem. Commun., 1978, 800–801 (1978)
3. [254] A. Furusaki et al., Bull. Chem. Soc. Jpn., 55, 3681–3685 (1982)
4. [540] N. Funato et al., Tetrahedron Lett., 35, 1251–1254 (1994)
5. [572] Y. Takahashi et al., Actinomycetol., 9, 19–26 (1995)
6. T. Tamaoki et al., Biochem. Biophys. Res. Commun., 135, 397–402 (1986)
7. J. T. Link et al., J. Am. Chem. Soc., 117, 552–553 (1995)
8. [474] Y. Sasaki et al., Eur. J. Pharmacol., 202, 367–372 (1991)
9. [749] Z. Li et al., J. Antibiot., 53, 426–429 (2000)
10. G. Caravatti et al., Med. Chem. Lett., 4, 399-404 (1994)


